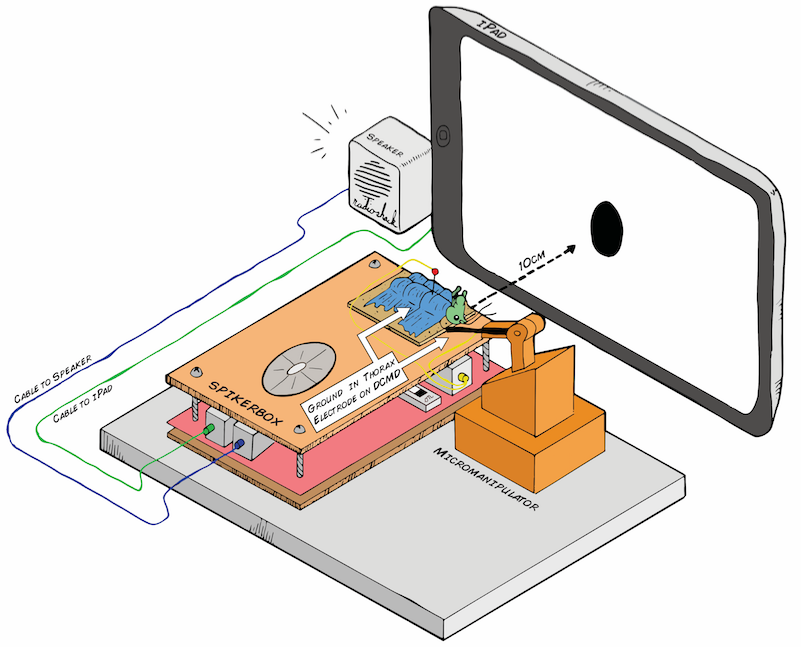
Below is the preliminary design for my electrophysiology setup. The Backyard Brains SpikerBox has a piece of cardboard on top, so after being anesthetized in ice, the little grasshopper will chill out there for the experiment, which would last about an hour. The grasshopper's belly (ventral side) would face up, so I can place the recording electrode in its neck (where the activity of the DCMD neurons can be picked up) and the reference in either its abdomen or thorax, where I assume the DCMD activity would not be found. Because the DCMDs are theoretically responsive to "contralateral" stimuli that is on the opposite eye, if I place the electrode (using the Backyard Brains 3D printed micromanipulator for precision) on the right side of the grasshopper's neck, I would expose its left eye to the iPad. I will use a level ruler to make sure the angle between the center of the screen (also the center of the stimulus) and the center of the grasshopper's eye is as minimal as possible.
Art by Tanner @ All Hands Active, MI
During the trials, the iPad screen will throw a black ball (in a white background for stark contrast) at the grasshopper's eye. Some parameters I can control are: distance between the ball/screen and the eye, the size of the ball, and the ball's velocity of approach (ball expanding in size). What will happen when a small ball is approaching the eye slowly? When it is fast? When the ball is big? We'll see!
The profile page of this project includes the full list of the materials I need and the detailed instructions, from preparing the little grasshoppers for the experiments to throwing simulated balls at them to recording their DCMD activity.
 Dieu My Nguyen
Dieu My Nguyen
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.