From a practical perspective, designing a galvanic cell (or bank of cells) is extremely easy. They can be treated like batteries because they are like batteries. For the purpose of this log (and project in general) I am going to describe everything in plain language instead of scientific terminology. If you want to deep dive into electrochemistry Google is your friend.
1) Pick anodes and cathodes that maximize difference in reactivity. The metals (or the like) you choose will determine the voltage produced by your cell. To determine what voltage your anode and cathode will produce, consult a reactivity table like below:
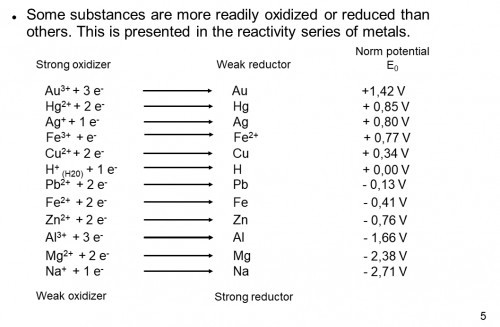
You want to pair a reactive metal (negative electric potential in the chart) with an unreactive metal (positive electric potential in the chart). The maximum voltage produced by your galvanic cell will the sum of reactivity.
For a copper-zinc cell the ideal voltage will be 0.34V(Cu) + 0.76V(Zn) = 1V
Lithium is the most reactive metal easily incorporated into a battery, which is why it produces such high power density batteries.
2) Pick a highly acidic electrolyte. The actual voltage produced by a galvanic cell will never equal its ideal voltage. How close you get depends largely on how good your electrolyte is. The role of the electrolyte is to transport ions. In general, strong acids are the best electrolytes because, in general, they can pack the most ions.
3) Maximize the surface area (and probably mass) of your annode and cathode to maximize available current. Although the voltage of your cell is determined by the reactivity of the annode and cathode, the current supplied by the cell is determined by the surface area of the annode and cathode exposed to the electrolyte. You want an electrolyte that can transport as many ions as possible, but this is still limited by the availability of ions (annode/Zn) and places to put ions (cathode/Cu). More ions means more current.
4) Although voltage of a single cell is limited by annode/cathode reactivity, voltage can be increased by placing cells in parallel - just like batteries. However, cells have to be isolated otherwise you will get a short between cells. To put it more specifically, an ion barrier between cells is necessary if they are adjescent in an electrolyte.
Pretty cool stuff.

In all the cells I've built I have sandwiched multiple strips of metal for each anode and cathode (in the above picture you can see how I've sandwiched Magnesium and Copper) in an attempt to maximize surface area, and therefore maximize available current.
 Curt White
Curt White
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.
Yeah, its pretty..... hackish ;) Do you know what the reaction potentials are in 0.16M HCL?
Are you sure? yes | no
you'll want to look at Nernst's equation to figure that out..
Are you sure? yes | no
Without any Cu²⁺-ions in solution and with an acidic electrolyte your cells are not Zn/Cu or Mg/Cu but rather Zn/H and Mg/H. The standard reduction potentials you show in the table are ..well, standard, meaning all concentrations are 1M (and pressure, if relevant, is 1 bar). The standard reduction potential for hydrogen is usually measured with a standard hydrogen electrode (SHE). In theory the value shouldn't change with a copper electrode, but I don't know if that is actually the case. Maybe there's an experienced electrochemist out there who knows..
Are you sure? yes | no