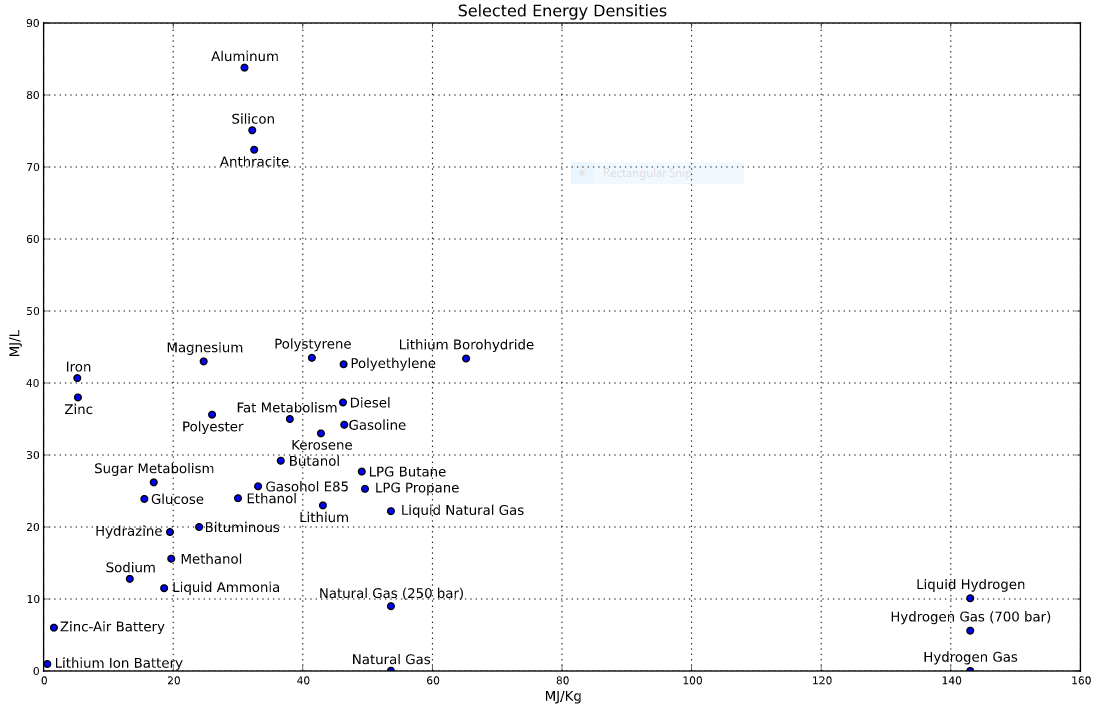
Batteries suck. Even the best batteries have woefully small energy densities, either on a MegaJoules/ Liter (volume) or MegaJoules/ kilogram (weight) basis. Most chemical fuels, on the other hand, have energy densities many magnitudes higher. Consider how far you can walk on the calories of a sandwich, a granola bar, or a sugary beverage. That's a lot of energy in a small package. See this figure. If you can't find lithium batteries, look towards the origin.
My cellphone is powered by a lithium battery that is recharged by a 5 volt adapter plugged into a 120 VAC line. This comes from a transformer that goes to a high-tension power line to a large generator which (in my area) is powered by gasoline or coal. (BTW, anthracite coal has one of the highest energy densities per unit volume, which makes it a terrific compact fuel for things like steam trains.) Any time a battery is charged, half of the energy is wasted as heat. It just is. There are other losses associated with the transmission of power. By the time it gets to my phone, the overall efficiency is-- well, not great. Could I do things more, uh, directly?
This battery powers my cellphone for about 14 hours under normal use. And then there are days where the battery only lasts half a day. This is frustrating for me. Sure, I could change a bank of lithium batteries, but just look at the chart!
Energy is all around me. I mentally explored a large solution space, including solar, wind, human powered (very promising), glucose fuel cells (have a sip of Gatorade, phone!) and various generators. Although each of these are fascinating and worthy of exploration, I eventually became enamored of a gasoline- or diesel- fueled solution.
Although I love the idea of recharging my cellphone with a small engine such as an RC airplane motor or a string trimmer engine, I had trouble visualizing those things indoors. Smoke and noise would make these, uh, offensive.
I eventually settled on the Zippo hand-warmer system as being one to emulate. Zippo-style hand warmers [INSERT LINK OR PIC HERE] generate mellow heat by "burning" lighter fluid without really burning it. Instead, the lighter fluid is oxidized at sub-flame temperatures by a catalyst, generally a finely-powdered metal (like platinum) on a mineral fiber substrate ("rockwool"). Heating this up to a modest temperature (usually by brief exposure to a flame) starts the catalysis, and the fuel-air mixture gets oxidized without forming actual fire. They emit fumes, but no soot or smoke.
A thermoelectric generator (TEG) use the Seebeck effect [LINK?] to directly generate electricity from a difference in temperature. Yeah, it's not a Stirling engine, but it could be considered a sort of solid-state heat engine. They are, sadly, only about 5% to 8% efficient. BUT they are solid state and quiet, with no moving parts and no noisy explosions.
Another drawback to TEGs is that reasonably priced ones fall to pieces at temperatures above their solder melting temperature, which is only slightly above 200 degrees C. Even more reasonably priced ones (which are really Peltier cooling elements sold as TEGs by the unscrupulous) fall apart at 150 degrees C, right about the point where you start getting significant power.
"Candle generators" exist, where a tealight or other flame directly powers a TEG to light LEDs or whatever. This will damage a TEG almost immediately, unless it's one of the pricey high-temp ones or something kludged from iron and copper, etc. My guess from looking at diagrams of a commercially available one is that heat sinks dump heat to reduce the temperature low enough to avoid damage to the TEG.
On the other hand, the "hand warmer" produces a steady heat well below flame temperatures, a good match for a sensitive TEG that needs to be hot but not TOO hot.
My device is a kludged-together apparatus that comprises (from bottom to top); a stainless steel hip flask filled with carbon felt as a fuel reservoir; a stainless steel housing that contains the catalytic material at a safe distance from the liquid fuel (such that only vapor gets to it); a TEG mounted on the housing; a heat sink mounted on the TEG to transfer heat to the air, creating a temperature difference. Electrically attached to the TEG is a regulator that bumps it up to 5 V and a USB port for charging my phone.
And it.. well, I'm not done yet, but we're getting there. The housing gets good and hot (around 175 - 200 degrees C) for several hours without catching itself and anything else on fire. More in the blog entries later.
 bryan.lowder
bryan.lowder