Glow in the dark stars contain a phosphorescent pigment consisting of copper doped zinc sulfide (ZnS:Cu) or europium doped strontium aluminate (SrAl2O₄:Eu).
It's super-easy to produce zinc sulfide, one simply mixes solutions of sodium sulfide and a soluble zinc salt e.g. zinc chloride:
However, merely precipitating ZnS in the presence of Cu²⁺-ions does not yield phosphorescent ZnS:Cu. Zinc sulfide formed this way has a zinc blende structure (it is zinc blende) and it has a crystal lattice as shown here (image stolen from wikipedia):
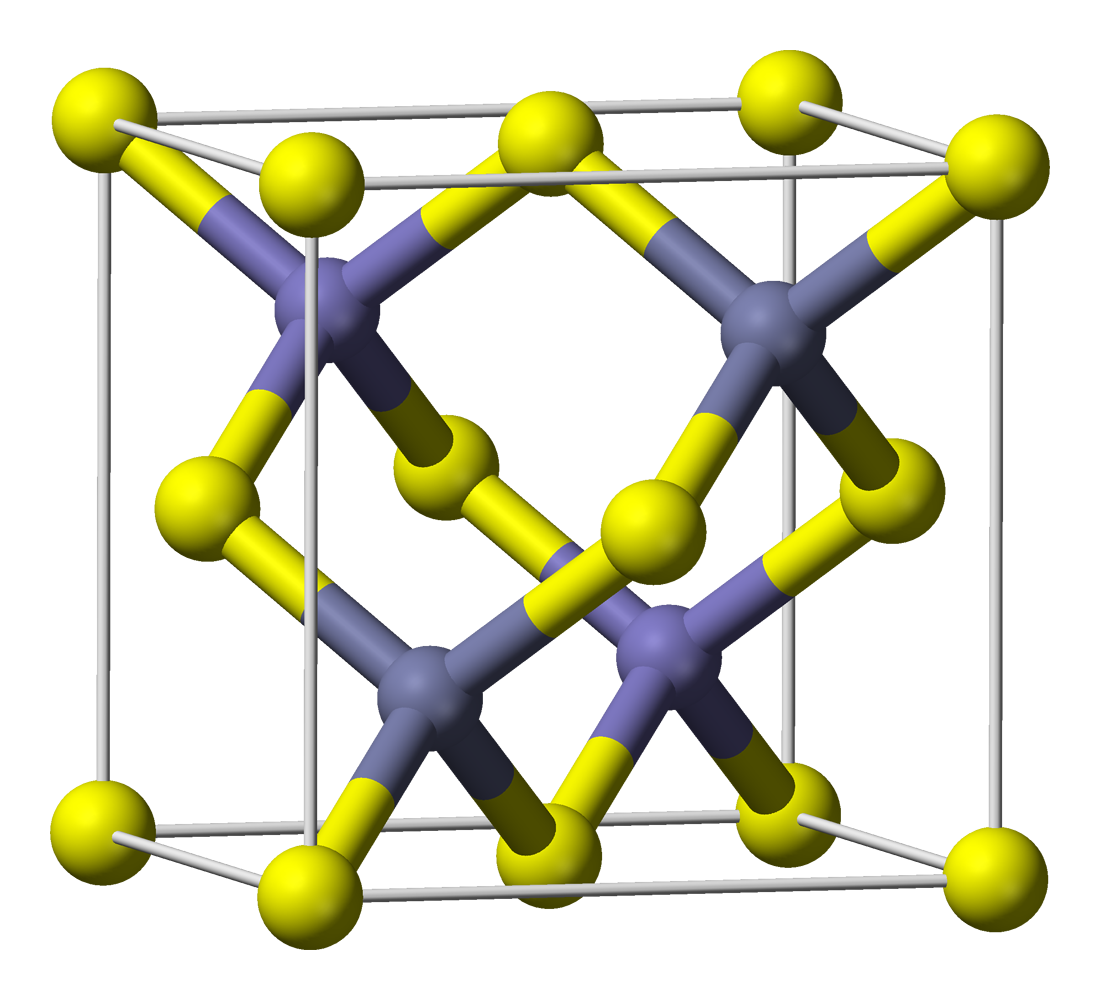
The phosphorescent ZnS:Cu is first formed when the ZnS/CuS is heated to app 900°C under an inert atmosphere (or better yet, in an atmosphere of H₂S). In other words, it's not for kids.
Zinc sulfide is a semiconductor and when doped with Cu, electron holes are introduced. When exposed to (near-)UV light electrons are excited to the conduction band, and when they relax and recombine with the holes, light is given off.
The kinetics of the decay can be studied by following the light intensity over time. In this experiment the Linear CCD module's SPI firmware was modified to power an LED (Osram Duris E5) which was placed behind a piece of a glow-in-the-dark star situated just in front of the CCD. The LED was turned off immediately (app 7.5 ms) before data collection.
The following graph shows the light intensity as a function of time:
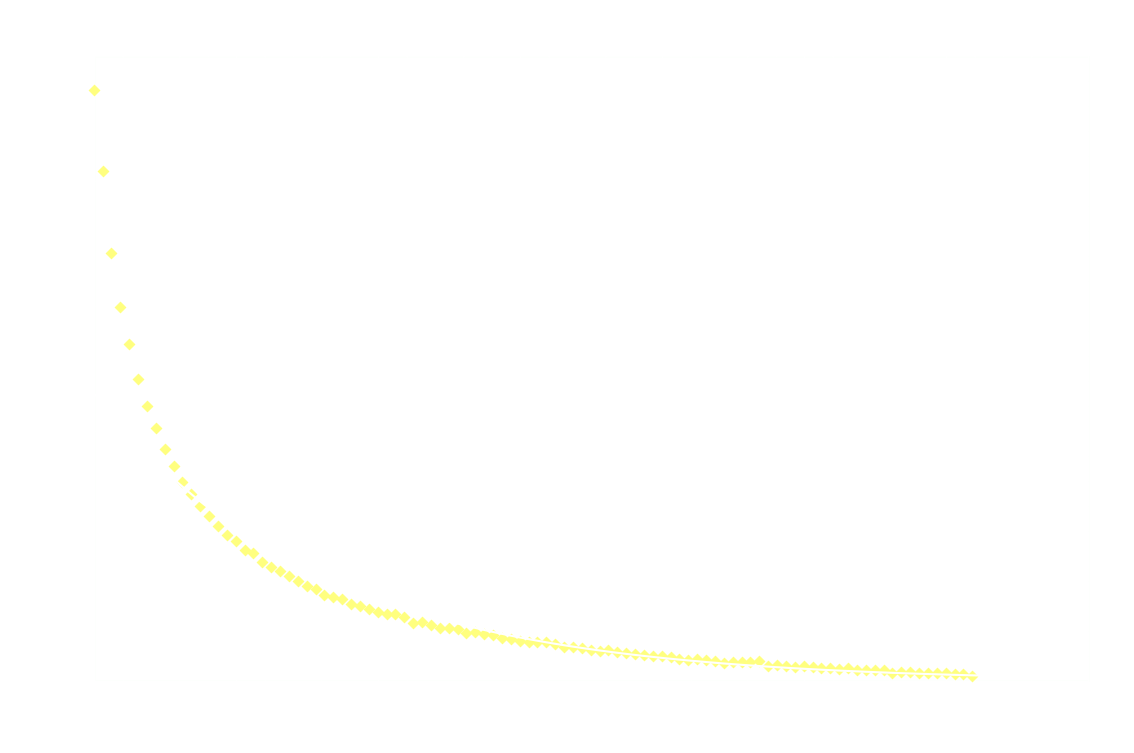
Surprisingly, the decay doesn't follow 1st order kinetics. The first 250ms it appears to follow 2nd order kinetics, as seen in the next graph:
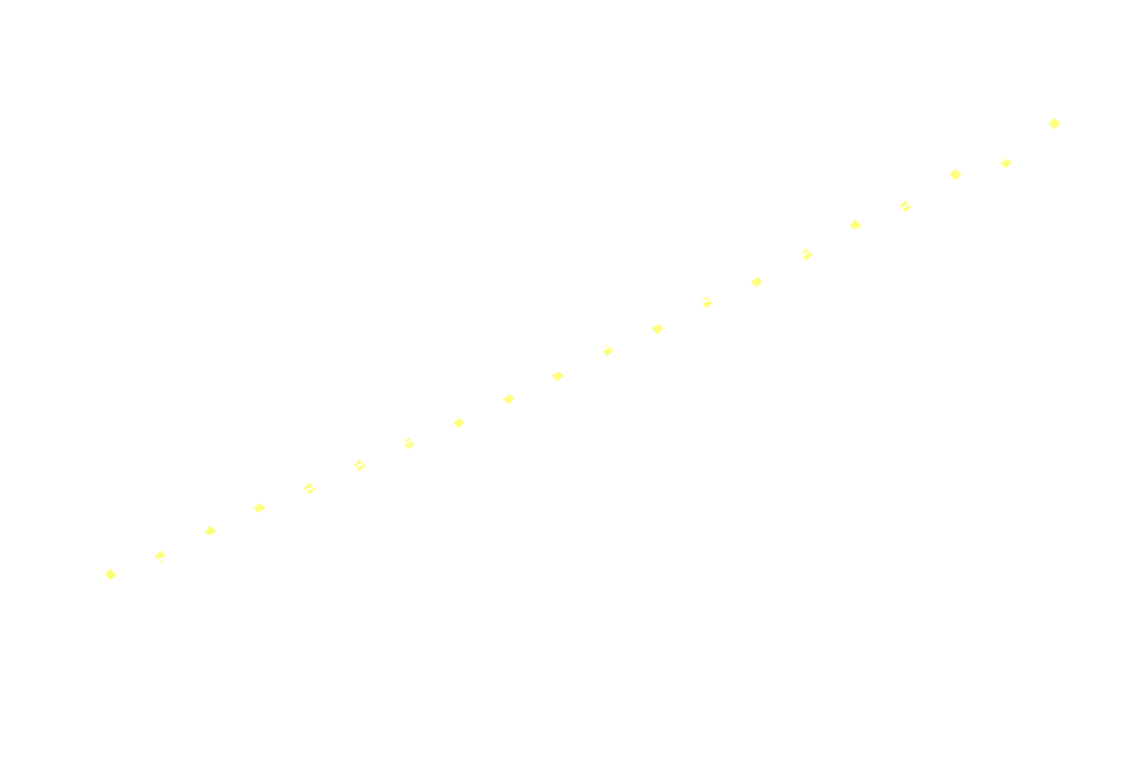
Hereon after, it's back to 1st order:
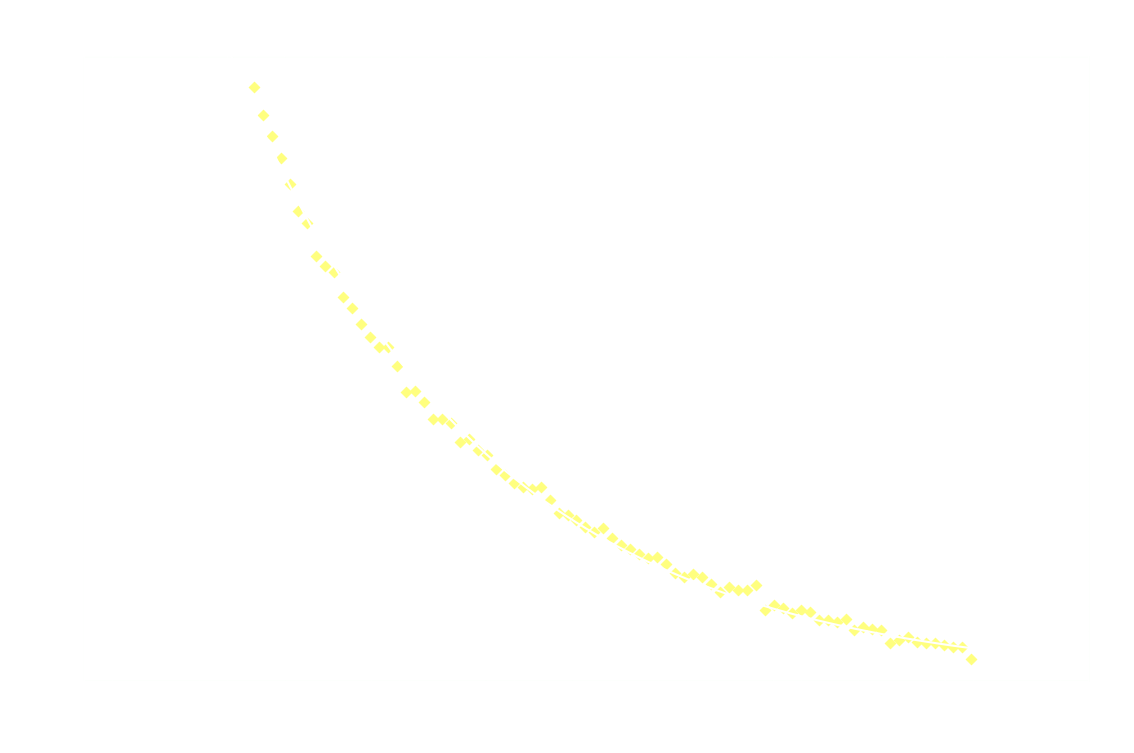
And just for good measure, here's the 2nd order model on the entire dataset:
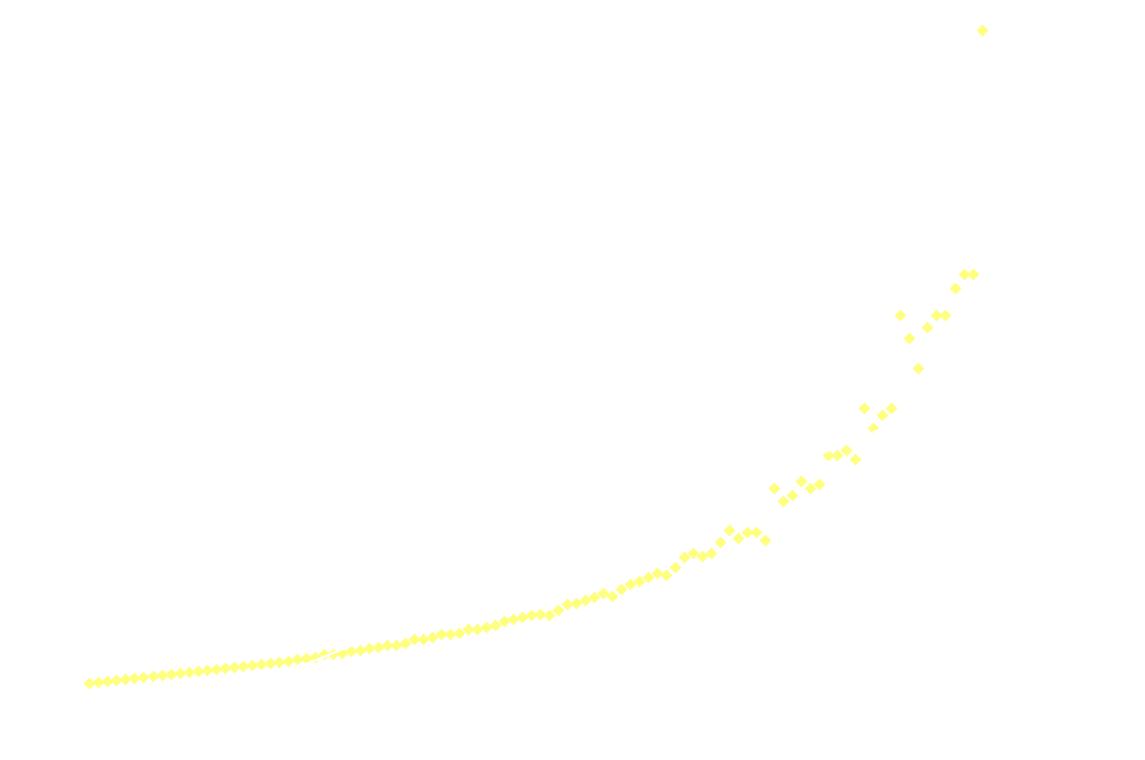
Clearly 2nd order is not a good fit after the first 250ms.
It's not the LED-phosphor playing tricks, the LED-emission stops almost instantaneously after it's powered off (at least it's so fast, that it doesn't interfere with the measurements).
A similar investigation is presented in the following article, but the authors reach the opposite conclusion, that the decay is 1st order initially, and then becomes 2nd order:
Experiments with Glow-in-the-Dark Toys: Kinetics of Doped ZnS Phosphorescence, George C. Lisensky, Manish N. Patel, and Megan L. Reich, J. Chem. Educ., 1996, 73 (11), p 1048
 esben rossel
esben rossel
Discussions
Become a Hackaday.io Member
Create an account to leave a comment. Already have an account? Log In.